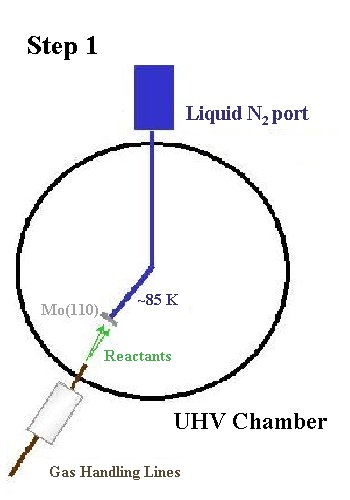
Temperature Programmed Desorption (TPD) is used to monitor desorption from the Mo(110) surface as a function of temperature using a mass spectrometer. The temperature is measured by a W-5% Re vs. W-26% Re thermocouple spot-welded to the edge of the crystal. When doing TPRS, the crystal is first cooled to 100 K with liquid nitrogen. A gaseous reactant is then dosed onto the crystal at 100 K. The exposure (torr·sec) is quantified by monitoring both the pressure rise in the chamber with an ionization gauge and also the dosing time.
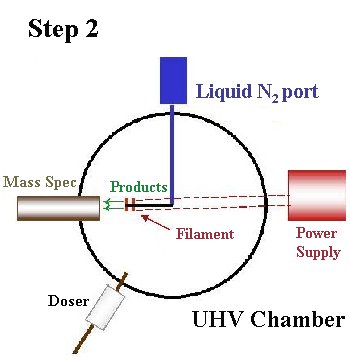
Typical adsorbate doses corresponded to pressure rises between 3×10-10 torr for 10 seconds and 2×10-9 torr for 5 minutes. Once the adsorbate is deposited, the crystal is rotated towards the UTI Model 100C quadrupole mass spectrometer, prior to heating.
Heating of the Mo(110) crystal up to 700 K is done via radiative heating using the tungsten filament positioned ~1mm behind the crystal with ~6 amps of current. To heat up to 2300 K, the crystal is positively biased (up to ~400V) so that electrons boiling off the filament during radiative heating bombard the crystal.
When performing TPRS experiments, the mass spectrometer is used to detect products desorbing from the crystal surface during heating. The spectrometer is enclosed by a shield with a 1/8" diameter aperture, to optimize detection of the molecules desorbing directly from the center of the crystal. The mass spectrometer signal of the gaseous desorption products is monitored as a function of both time and temperature using the TPRS98 program interfaced to the mass spectrometer and thermocouple. A linear heating rate of ~7 K/s from 100 K to 700 K is produced by the Eurotherm 906S controlling the Kepco ATE 6-10M. To prevent electron-induced reactions the crystal is negatively biased during these experiments. For TPRS experiments above 700 K, because the crystal is positively biased, a linear heating rate cannot be obtained.
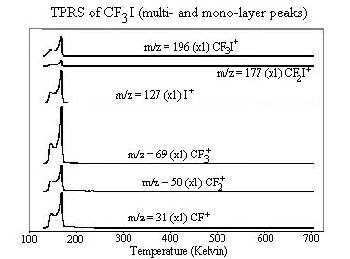
Fragmentation patterns from the mass spectrometer data are used to identify molecules desorbing from the surface. For example, a mass to charge ratio of 127 corresponds to iodine. The area under each desorption peak is proportional to the surface coverage. Integrating the desorption peaks provides relative product yields. The temperature at which a molecule desorbs also provides information about the surface-molecule interactions. In general, the higher the desorption temperature, the more tightly bound a molecule is to the surface.